Research focus
The focus of the laboratory is on developing human organoid models of diseases affecting the nervous system. This allows to uncover early, cell type-specific pathomechanisms. Understanding these can help to identify drug targets for treatment and developing effective intervention therapies.
The laboratory is specifically interested in amyotrophic lateral sclerosis (ALS), a currently incurable neurodegenerative disease that affects motor neurons and glial cells of the primary motor cortex and spinal cord. ALS leads to a complete loss of muscle control and death within 3-5 years. For disease modelling purposes it is necessary to use models of the area affected by the disease therefore, the members of the laboratory are developing protocols for the generation of cortical and spinal cord organoids from human induced pluripotent stem cells (hiPSCs). Human iPSCs can be obtained from healthy donors or patients suffering from ALS leading to the unique possibility of recapitulating the genetic background of the disease as well as some of the early disease phenotypes in region-specific ALS organoid models.
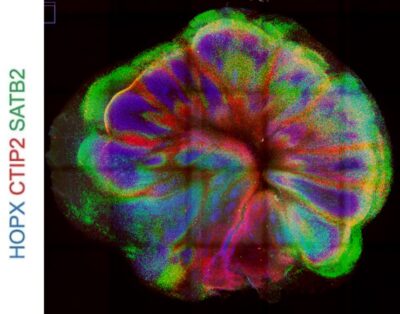
100 days old organoid slice with radial glia (HOPX), cortical layer II-IV (SATB2) and layer IV-VI neurons (CTIP2).
Courtesy of Lea Wenger, modified from Szebényi et al., Nat Neurosci 2021.
Cells differentiating in organoids can become more mature than their counterparts created under two-dimensional culture conditions, leading to a higher ability to reveal disease phenotypes. The inclusion of organoids in drug development processes is therefore an extremely important task, but using three-dimensional tissues for high-throughput systems is still hindered by a few technical difficulties. With the help of a procedure developed by the principal investigator, certain cell types can be selected from the organoid models and propagated as two-dimensional cell cultures. These cultures are used by the laboratory currently to develop a high-throughput drug screening platform.
Members of the lab are also interested in understanding the molecular mechanisms of cerebellar ataxias. Patients suffering from various types of spinocerebellar ataxias become severely limited in their movements, which is caused by pathological changes in one specific type of neurons in the cerebellum, the Purkinje neurons. To this end cerebellar organoid protocols are developed and iPSCs carrying ataxia causing mutations are used for disease modelling.
In cooperation with the Drug Resistance group of the Research Institute for Natural Sciences and with the 2nd Department of Pediatrics, Semmelweis University, the laboratory also investigates neuroblastoma, a malignant tumour of the sympathetic nervous system, arising in the derivatives of the neural crest. Neuroblastoma mainly affects children under the age of 5 and is the most common cancer in infants. High-risk neuroblastoma is often fatal due to the resistance to therapy that develops during treatment. To be able to discover the underlying mechanisms protocols for neural crest organoid differentiation are developed.
Lab members
Kornélia Szebényi, principal investigator (szebenyi.kornelia@ttk.hu)
Éva Bakos, senior researcher
Flóra Vajda, postdoctoral researcher
Alexandra Fejes, PhD student
Simon Tusnády, MD-PhD student
Julianna Pap, Scientific Student’s Association Student
Krisztina Mohos, lab manager
Krisztina Dolniczki, research assistant
Funding
Szebényi, Kornélia
¤ IBRO Return Home Fellowship 2022
¤ HORIZON-WIDERA-2022-TALENTS-02-01
¤ Hungarian Scientific Research Fund (FK OTKA)
¤ Hungarian Scientific Research Fund (STARTING)
¤ ALS Finding A Cure / Hop On A Cure
¤ János Bolyai Research Fellowship
¤ New National Excellence Programme (Hungarian Scientific Research Fund)
¤ Cooperative Doctoral Programme (Hungarian Scientific Research Fund)
¤ Pannonia Scholarship Programme
Tusnády, Simon
¤ New National Excellence Programme (Hungarian Scientific Research Fund)
¤ Kerpel Scholarship
¤ Cooperative Doctoral Programme (Hungarian Scientific Research Fund)
¤ Scholarship from Richter Gedeon Centenarium Foundation
Collaborations
Human Pluripotent Stem Cell Laboratory, Research Centre of Natural Sciences, Budapest, Hungary
Drug Resistance Research Group, Research Centre of Natural Sciences, Budapest, Hungary
2nd Department of Pediatrics, Semmelweis University, Budapest, Hungary
Lakatos Lab, Department of Clinical Neuroscience, University of Cambridge, Cambridge, United Kingdom
Ivics Lab, Clinical Gene Transfer Department, Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany
Selected publications
Nie Y, Szebényi K, Wenger LMD, Lakatos A, Chinnery PF. Origin and cell type specificity of mitochondrial DNA mutations in C9ORF72 ALS-FTLD human brain organoids. Sci Adv. 2025;11(10):eadr0690. doi:10.1126/sciadv.adr0690
Szebényi K, Vargova I, Petrova V, Tureckova J, Gibbons G, Rehorova M, Abdelgawad M, Sandor A, Marekova D, Kwok J, Jendelova P, Fawcett J, Lakatos A. Inhibition of PHLDA3 expression in human SOD1 amyotrophic lateral sclerosis astrocytes protects against neurotoxicity. Brain Commun. 2024;6(4):fcae244. Published 2024 Jul 25. doi:10.1093/braincomms/fcae244
Szebényi K, Füredi A, Bajtai E, Sama SN, Csiszar A, Gombos B, Szabó P, Grusch M, Szakács G. Effective targeting of breast cancer by the inhibition of P-glycoprotein mediated removal of toxic lipid peroxidation byproducts from drug tolerant persister cells. Drug Resist Updat. 2023;71:101007. doi:10.1016/j.drup.2023.101007
Szebényi K, Barrio-Hernandez I, Gibbons GM, Biasetti L, Troakes C, Beltrao P, Lakatos A. A human proteogenomic-cellular framework identifies KIF5A as a modulator of astrocyte process integrity with relevance to ALS. Commun Biol. 2023 Jun 29;6(1):678. doi: 10.1038/s42003-023-05041-4. PMID: 37386082; PMCID: PMC10310856.
Szebényi K, Wenger LMD, Sun Y, Dunn AWE, Limegrover CA, Gibbons GM, Conci E, Paulsen O, Mierau SB, Balmus G, Lakatos A. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat Neurosci. 2021 Nov;24(11):1542-1554. doi: 10.1038/s41593-021-00923-4. Epub 2021 Oct 21. PMID: 34675437; PMCID: PMC8553627.
Szabó Z, Héja L, Szalay G, Kékesi O, Füredi A, Szebényi K, Dobolyi Á, Orbán TI, Kolacsek O, Tompa T, Miskolczy Z, Biczók L, Rózsa B, Sarkadi B, Kardos J. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci Rep. 2017 Jul 20;7(1):6018. doi: 10.1038/s41598-017-06073-7. PMID: 28729692; PMCID: PMC5519671.
Szebényi K, Füredi A, Kolacsek O, Pergel E, Bősze Z, Bender B, Vajdovich P, Tóvári J, Homolya L, Szakács G, Héja L, Enyedi Á, Sarkadi B, Apáti Á, Orbán TI. Generation of a Homozygous Transgenic Rat Strain Stably Expressing a Calcium Sensor Protein for Direct Examination of Calcium Signaling. Sci Rep. 2015 Aug 3;5:12645. doi: 10.1038/srep12645. PMID: 26234466; PMCID: PMC4522653.
Szebényi K, Füredi A, Kolacsek O, Csohány R, Prókai Á, Kis-Petik K, Szabó A, Bősze Z, Bender B, Tóvári J, Enyedi Á, Orbán TI, Apáti Á, Sarkadi B. Visualization of Calcium Dynamics in Kidney Proximal Tubules. J Am Soc Nephrol. 2015 Nov;26(11):2731-40. doi: 10.1681/ASN.2014070705. Epub 2015 Mar 18. PMID: 25788535; PMCID: PMC4625667.
Szebényi K, Péntek A, Erdei Z, Várady G, Orbán TI, Sarkadi B, Apáti Á. Efficient generation of human embryonic stem cell-derived cardiac progenitors based on tissue-specific enhanced green fluorescence protein expression. Tissue Eng Part C Methods. 2015 Jan;21(1):35-45. doi: 10.1089/ten.TEC.2013.0646. PMID: 24734786; PMCID: PMC4291086.
Photos




